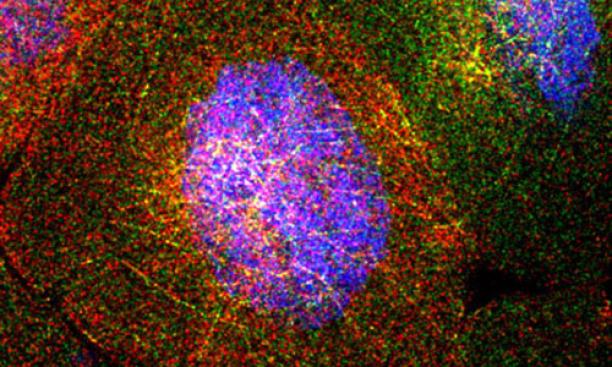
The fruit fly ovary consists of about 100 egg chambers. Each chamber contains 15 "nurse cells." These surround the oocyte, or egg cell, which ultimately will develop into a baby fruit fly. The nurse cells synthesize RNA molecules that are ultimately deposited into the oocyte. This image, from a collaboration of Princeton's Gavis and Wieschaus labs, shows four nurse cells. Each red or green dot is an individual RNA molecule, which is produced from DNA (shown in blue). The RNA molecules intermingle on a threadlike network that allows them to move from one nurse cell to another and then into the developing egg (which we don't see in this image). This image earned the People's First Place prize in Princeton's 2013 Art of Science competition.
Courtesy Shawn C. Little, Kristina S. Sinsimer, Elizabeth R. Gavis, and Eric F. Wieschaus
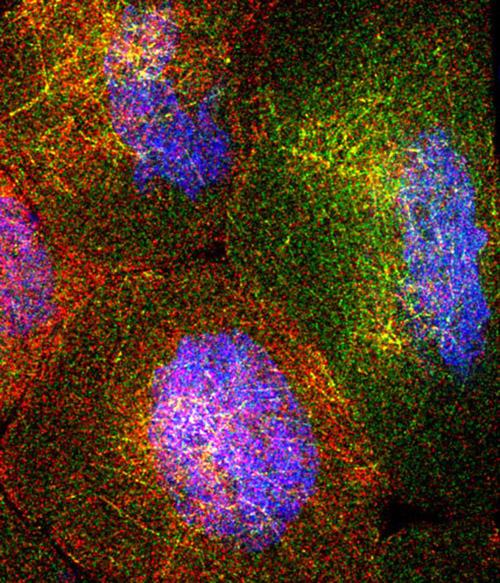
The fruit fly ovary consists of about 100 egg chambers. Each chamber contains 15 "nurse cells." These surround the oocyte, or egg cell, which ultimately will develop into a baby fruit fly. The nurse cells synthesize RNA molecules that are ultimately deposited into the oocyte. This image, from a collaboration of Princeton's Gavis and Wieschaus labs, shows four nurse cells. Each red or green dot is an individual RNA molecule, which is produced from DNA (shown in blue). The RNA molecules intermingle on a threadlike network that allows them to move from one nurse cell to another and then into the developing egg (which we don't see in this image). This image earned the People's First Place prize in Princeton's 2013 Art of Science competition.
Courtesy Shawn C. Little, Kristina S. Sinsimer, Elizabeth R. Gavis, and Eric F. Wieschaus
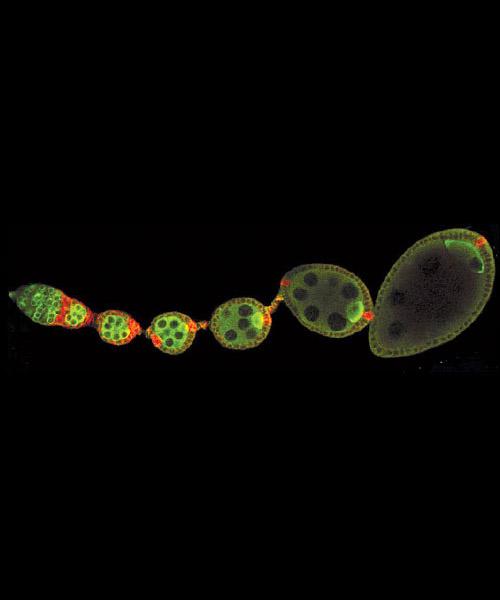
Fertilization of the fruit-fly egg kicks off the complicated process of forming an embryo. Molecular biology professor Gertrud (Trudi) Schüpbach studies the genetic and molecular processes that underlie this development. The image above shows wildtype egg chambers developing inside an ovariole.
Courtesy Gertrud M. Schüpbach
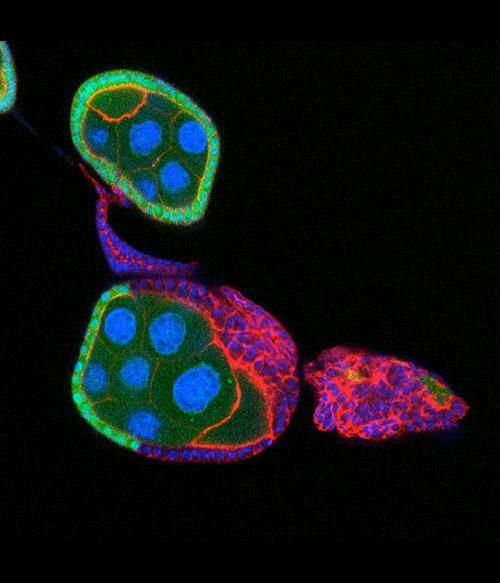
Schüpbach's work also explores mutations that affect the embryo. In this image, a normal egg chamber on top has a single layer of wildtype (green) follicle cells surrounding the nurse cells and oocyte. In the egg chamber at the bottom, the mutant follicle cells (red with blue nuclei) have overgrown and formed a tumor.
Courtesy Gertrud M. Schüpbach
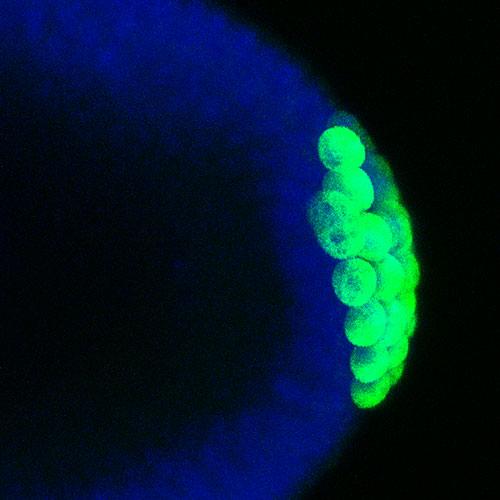
As the fruit fly embryo develops, a special group cells can be seen pinching off at the posterior end. Later on, these cells - the germ cells – will produce the eggs or sperm of the animal. The germ cells form only at the posterior of the embryo under the instruction of a group of mRNA and protein molecules that are located specifically at the posterior. Many of these molecules, like the one that can be seen in green in this image, are put into the germ cells as they pinch off and are important for their further development. Professor Elizabeth Gavis’ lab has been studying how these mRNA and proteins become located at the posterior of the embryo and how they are inherited by the germ cells in order to produce fertile animals.
Courtesy Elizabeth R. Gavis
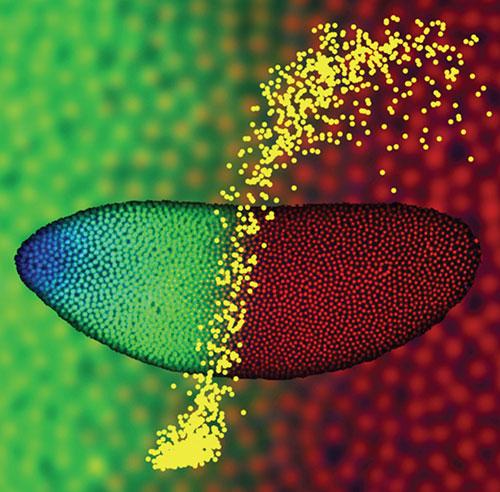
The image shows a Drosophila embryo two hours after fertilization, with nuclei at the surface fluorescently labeled for Bicoid protein (blue), Hunchback protein (green), and DNA (red). Using two-photon microscopy these embryos were imaged to quantitatively characterize the dynamics and precision of how morphogen molecules communicate positional information to individual nuclei. In this example, the shallow Bicoid gradient generates a sharp Hunchback boundary (enlarged in the background), partitioning the embryo in half. This input/output relationship is quantitatively represented in the foreground (yellow), where each dot specifies the Bicoid concentration (horizontal axis) and Hunchback concentration (vertical axis) measured in a single nucleus. The results indicate that the precision with which the embryo interprets and locates this boundary is very high, approaching limits set by simple physical principles.
Courtesy Thomas Gregor
