
Asymmetry is beautiful We depict the heart as a symmetrical shape on Valentine’s Day cards, but the heart actually starts out in the developing fetus as a tube. That symmetry is broken when the heart migrates to the left side of the body. Rebecca Burdine, an associate professor of molecular biology, has been working to understand how a crucial protein called Nodal helps break the body’s initial symmetry, guiding the asymmetrical development and positioning of the organs, including the heart.
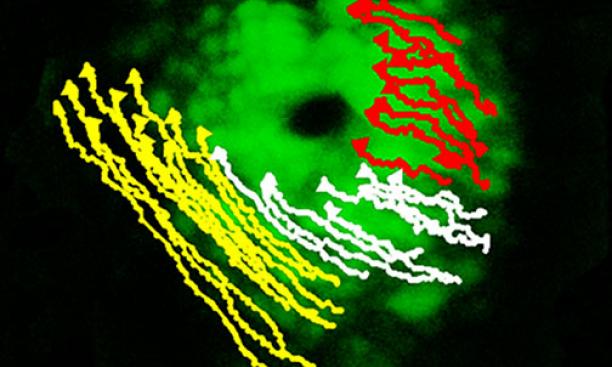
Heart of a fish Burdine’s laboratory observes the development of the heart in zebrafish embryos, which are transparent and undergo development more quickly than laboratory mice. Researchers film the two-hour heart development under a microscope and watch as it becomes asymmetric, comparing normal and mutated zebrafish embryos to tease out which genes are important and how.
According to Martin Blum, a professor at the University of Hohenheim in Stuttgart, Germany, Burdine was among the first to show that the basic steps of correct asymmetrical development are the same in all animals, from simple vertebrates to humans, and have remained the same through evolution — the way the heart develops in zebrafish is not that different from what occurs in people.

It all starts at the node The breaking of symmetry likely starts at a hairy structure in the middle of the embryo called the node, biologists have shown. The tiny hairs, called cilia, move quickly, creating flow and pushing extra-cellular molecules in one direction, likely guiding the positioning of developing organs. This flow results in a concentration of the Nodal protein on the left side of the embryo. But the puzzle is not yet solved: In zebrafish, the flow of molecules is like the movement of clothes in a dryer, spinning in one direction. It is not easy to see how this movement can distribute the molecules asymmetrically, Burdine said.
She recently showed that of the two populations of cells that first come together to make the initial heart tube, the population exposed to the Nodal protein moves much faster than the other. But because both cell groups are connected, the speedy ones drag the slow cells, creating a cone shape that eventually orients itself on the left side of the axis and forms two different sides of the heart.
The research identified important genes necessary to break the heart’s symmetry and suggests how combinations, rather than single genes, may lead to congenital heart defects. “I am really interested in how our research impacts human health,” Burdine said.
