
The Toolmaker’s Mind
Clifford Brangwynne marries engineering and biology in a quest to understand the inner workings of the cell
The best way to learn how intensely Princeton bioengineer Clifford P. Brangwynne approaches life is to catch him playing hockey at Baker Rink. Before the COVID-19 pandemic, he had a standing noontime game. He’s played since childhood, mostly defense. At Baker, he might be fending off collaborator and friend Mikko Haataja, a professor of mechanical and aerospace engineering at Princeton and a formidable forward. “When you play intense sports, that’s the only thing that’s consciously on your mind,” Brangwynne says. With skates on, he gets in the zone. And when Cliff Brangwynne gets in the zone, there’s no stopping him.
Brangwynne, the June K. Wu ’92 Professor of Chemical and Biological Engineering, an investigator at the Howard Hughes Medical Institute, and a MacArthur “genius grant” recipient, usually is in the zone scientifically, too. In 2009, when he was a postdoctoral researcher, he published a major finding, something fundamental about how cells are organized. In textbook diagrams, cells look tidy. Little subcompartments called organelles, each surrounded by a membrane, do jobs like storing genetic information or producing energy. But Brangwynne found an organelle that was not bound by a membrane. Instead, it acted like a lava lamp or oil-and-vinegar salad dressing. When he looked under a microscope, he saw liquid blobs fusing and breaking apart within a cell.
Within a few years, scientists started spotting lava-lamp behavior all over all kinds of cells, including human cells. Today, they know that the blobs, called droplets or biomolecular condensates, form when certain proteins in the cell crowd together in a temporary way. It’s akin to the way people in the internet phenomenon known as a flash mob rapidly assemble in a public place; perform some act, like a dance number; and just as suddenly, disperse. Work from several labs has implicated malfunctioning cellular droplets in diseases such as Alzheimer’s and ALS (Lou Gehrig’s disease).
Yet Brangwynne saw challenges looming. It’s one thing to be able to see droplets in cells. It’s another to know the rules that govern their formation, or to know all the purposes they serve. To answer those questions, he’d need to go beyond passive observation. He would have to learn how to control droplets, because controlling something demonstrates that you understand it. “Cliff’s got a great nose for exciting problems,” Haataja says. And it was in response to this problem, Haataja says, that “the bioengineer side of Cliff took over.”
Bioengineering is a big word that covers a big swath of science. Bioengineers, Brangwynne says, use ideas from quantitative fields to study biology. They mobilize the components of biology, like proteins, and wield them the way a construction worker would a drill. They build a toolkit to answer fundamental questions about biology or to solve a vexing problem for society. It’s an approach that has already taken Princetonians far.
Driven by a desire to reduce pollution, Frances Arnold ’79 recognized the environmental cost of producing modern conveniences like medications and fuels. She set out to coax proteins to make them without all the waste. When she launched her lab at the California Institute of Technology in the 1980s, scientists contended that brainpower and computer power would reveal precise instructions for making proteins do their bidding. But proteins contain hundreds or thousands of amino acids. With 20 amino acids to choose from, that works out to a daunting number of possible combinations. Arnold didn’t like those odds, so she tried harnessing biology’s way of tweaking proteins — evolution — to create a protein-customizing tool any scientist could use. Today, manufacturers of drugs and laundry detergent alike use her technique, known as directed evolution. She took home a share of the 2018 Nobel Prize in Chemistry.
In 1961, when Osamu Shimomura was a researcher in Princeton’s Department of Biology, he isolated a greenish, glowing protein from a jellyfish. But he didn’t stop there. He looked under the proverbial hood to see what made the protein glow. Other glowing proteins known at the time required chemical additives to light up, but Shimomura’s protein needed only a blue light source. Scientists seized the opportunity to use the green protein as a tool. Among countless applications, they used it to track how cancer cells spread and to detect arsenic in water wells. Shimomura earned part of the 2008 Nobel Prize in Chemistry. Later, in 2014, part of the chemistry Nobel went to a researcher who used the protein to bump up the resolution of light microscopes, which allowed scientists to see a cell’s minute subcompartments — including droplets — in sharper focus.
The more Brangwynne immersed himself in Princeton’s community of engineers, the more he realized that he, too, needed a new tool to be able to control or manipulate droplets in cells. “He’s always of the mindset that if there’s a controversy or if there’s something that’s unknown, it’s because we haven’t made the right measurement, and if we haven’t made the right measurement, it’s because we probably don’t have the right technology,” says Rohit Pappu, a longtime Brangwynne collaborator and a theoretical biophysicist at Washington University in St. Louis. In September 2015, Brangwynne landed a grant from the National Institutes of Health. In the section of the grant application that asks about the public-health reason for the funding, Brangwynne wrote, “we will develop a cutting-edge technology” to control and study droplets in ways that would be relevant to various cancers and other diseases.
At first, Brangwynne admits, “I don’t think we really understood what we were doing.” To control droplets on cue, he and his collaborators figured they’d have to start with the “flash mob” proteins that crowd together to form droplets, and then soup them up with other protein parts to create a remote control of sorts that would let a scientist cue the crowding. Finding the right parts was not trivial.
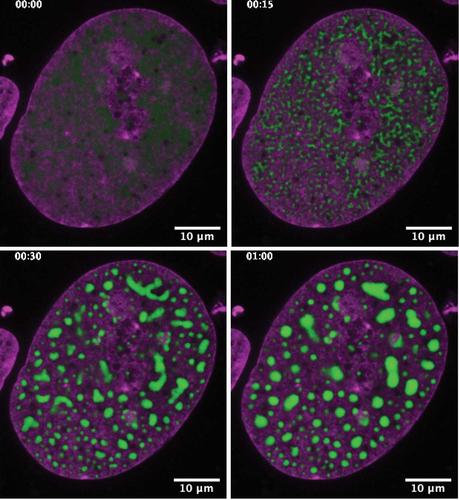
Fortunately, other scientists had built a thriving field around proteins that shape-shift or assemble in response to a flash of light. Laser light is a fantastic remote-control mechanism because it can be turned on or off instantly, and it is so focused that it can be shone on specific regions of a cell. So the researchers attached a light-responsive protein to a flash-mob protein, and then added a glowing protein so they could see their invention under a microscope. Then they put the whole shebang inside cells they had grown in petri dishes. “By coming up with this hybrid and shining a light, you nudge the system” and droplets form, says Haataja, a collaborator on the project. Wherever the team zapped their cells with a blue laser, presto, droplets formed.
As mind-boggling as it was to finally be able to manipulate droplets in a cell, something was still nagging at the bioengineer in Brangwynne. It was a numbers problem. In nature, Brangwynne says, the flash-mob proteins that drive droplet formation assemble in clusters that have the same number of partners every time. He was curious whether droplets would form with fewer or more partners, and what exactly was the threshold concentration for droplet formation. He couldn’t answer those questions and others like them, because the light-responsive protein the team chose didn’t cluster together in consistent numbers.
To Brangwynne, the droplet field has been all about demonstrating that the principles of physics that govern liquid flow in a lava lamp or salad dressing also apply to the microscopic milieu of the cell. Experts in areas as diverse as polymer physics and cell biology are studying droplets, and that can require bridging different scientific cultures to arrive at the truth, he says. Physicists and mathematicians state their hypotheses with numbers and equations, while biologists may use drawings or diagrams. Brangwynne is a physicist by training, and he thinks that as biology seeks to answer ever more complex questions in the 21st century, the field is going to need to integrate principles of mathematics or physics that perhaps weren’t as essential in the 20th.
Brangwynne “brings an engineering pragmatism to his thinking,” says Amy Gladfelter ’96, a Brangwynne collaborator and quantitative cell biologist at the University of North Carolina, Chapel Hill. “So much of engineering is about product and application, but he has this ability to use the principles of engineering for basic science discovery that is pretty unique as an individual or as a thinker.”
Brangwynne and his collaborators hunted for a protein that would consistently cluster with the same number of partners. They decided on ferritin, a protein found in blood, which assembles into a 24-unit cluster. The team again added a glowing protein to track things under a microscope — the glowing green protein Shimomura discovered at Princeton, in fact. They also added a light-responsive component, and finally, the flash-mob protein. It was a construction project on a molecular scale. Sure enough, with a flash of blue light, the new system formed droplets in cells on command. The team calls the technology “core scaffolds to promote droplets,” or Corelets for short.
With Corelets, it’s possible to begin tackling the quantitative questions that nagged at Brangwynne. The team has already created a quantitative “fingerprint” of the physical conditions that lead to droplet formation in cells. This fundamental information could someday help researchers understand how to intervene when droplets malfunction in diseases.
The tiny, lava-lamp-like droplets that form and break apart with Corelets technology are “mesmerizing to watch,” Gladfelter says. “They flow, and they drip, and they fuse. They do these things that are like what we see in our everyday lives. You pour maple syrup, or you’re making salad dressing and see droplets of oil. We’re used to these sorts of interactions with materials. And then to see it at a microscopic scale, it makes you feel like you’re in this invisible world,” she says. With Brangwynne’s technology, a scientist is making it all happen, controlling what’s going on in a cell almost as easily as if she were pointing and clicking a mouse. “There’s something very beautiful and satisfying about that,” Gladfelter says.
For José Avalos, Corelets’ flexibility is a selling point. Avalos, an assistant professor of chemical and biological engineering at Princeton, is a co-author on the Corelets work. He is interested in using droplets to transform single-celled organisms into tiny factories. To do this, his team is fusing yet more proteins — enzymes that could make pharmaceuticals or fuels in more sustainable ways — to Brangwynne’s systems. The original tool had forced Avalos to tack his enzymes onto one giant conglomerate of proteins. It was crowded from a molecular standpoint, and in that situation “some enzymes just aren’t going to be happy,” Avalos says. The Corelets system is structured differently, he says, and it’s possible to devise less-crowded versions that keep enzymes happy and functional.
In parallel to Corelets, Brangwynne and his collaborators developed yet another tool. This technique, called CasDrop, not only summons droplets at will, but it forms them at specific locations in the DNA inside lab-grown cells. The homing device that targets the droplets to DNA is a protein called Cas9, a component of the Nobel Prize-winning gene-editing system called CRISPR. This Cas9 is modified so that it retains its homing abilities but lacks the ability to snip DNA.
Amy Strom, a postdoctoral fellow in Brangwynne’s group, is using CasDrop to learn more about the interplay between droplets and the genome. The working hypothesis is that droplets have the capability to impact how a cell reads genetic instructions, to influence which genes get turned on or off. “I’m super excited about this technology,” Strom says. “We can ask new questions that we weren’t able to address before.”
Both Corelets and CasDrop are getting attention. Scientific American placed them among their top 10 emerging technologies of 2019. The scientific journal Nature named CasDrop a technology to watch in 2020. In December, Brangwynne gave a virtual lecture about the tools at a cell-biology conference. One enthralled scientist posted a reaction to the talk on Twitter. The tweet included an emoji with its mouth agape, a mushroom cloud emerging from its head. Translation: mind blown.
While Brangwynne’s lab was using a bioengineering approach to make droplets coalesce on cue, a bioengineering community was coalescing at Princeton. The Princeton Bioengineering Initiative launched in November, with Brangwynne at the helm. At a kickoff lecture during the virtual launch party, Brangwynne called plans for the initiative “unapologetically ambitious.” The community spans engineering and life-science departments, as well as physics and computer science. Fundraising and planning are underway for a building to act as a physical bioengineering hub. Princeton’s community-engagement campaign, “A Year of Forward Thinking,” launched last fall, featured a bioengineering theme in March. The initiative is recruiting its first crop of distinguished postdoctoral fellows. The call for applications reads: “We particularly welcome projects that seek to develop novel technologies.”
Brangwynne beams when he talks about bioengineering at Princeton. “It’s the field that’s going to transform humanity in this century,” he says. He hopes the bioengineering initiative will spark new collaborations between Princeton’s faculty and with New Jersey’s network of pharmaceutical and biotech companies. He dreams of next-generation medical devices, smarter tests that will fish out successful drugs faster, and solutions to problems like cancer or dementia.
He isn’t dreaming with blinders on, however. “We are very aware of the enormous ethical implications of some of the technologies that fall within bioengineering,” with CRISPR gene editing perhaps paramount among them, he says. At the same time, not every technology within bioengineering will provoke the same levels of concern, and as in any field, different technologies will require different degrees of forethought and caution when it comes to their application. “We are right now experiencing on an absolutely massive global scale, a huge success of bioengineering,” Brangwynne says, referring to the use of vaccines to engineer the human immune response to fight off COVID-19. “I think one has to be careful, and pragmatic, and realistic about where we’re drawing lines,” he says, because different people are going to have different comfort levels with the ethics of specific bioengineering technologies.
While planning for the bioengineering institute continues, the Brangwynne lab hums with activity. In July, Brangwynne was named a Blavatnik National Awards laureate — an honor that came with $250,000, the largest unrestricted science prize to young researchers. In October, the lab landed a highly competitive five-year, $7.5 million grant from the Department of Defense. Gladfelter, Avalos, and Pappu are among Brangwynne’s co-awardees. Together, they’ll use the funds to figure out ground rules for engineering droplets so that they can produce new and useful products. One possible application of this work is droplets designed to pump out advanced materials important for national security. Unrelated to all that, Brangwynne and others in the droplet field have launched drug startups to commercialize therapies for cancer and other diseases where droplets go awry.
Haataja, his collaborator and hockey adversary, has every confidence that Brangwynne will continue to succeed. “Cliff on the ice is similar to Cliff the scholar,” Haataja says. “He’s tenacious. He doesn’t give up. He pushes himself, but in a good way. And when he does something, he does it at 100 miles per hour.”
Carmen Drahl *07 is a Washington, D.C.-based writer whose work appears in Forbes and Scientific American.






No responses yet