Chemistry: Producing a New Molecular Bond
Two chemistry professors have developed a new type of reaction from two molecules, which could greatly speed up the work that leads to the discovery of new drugs.


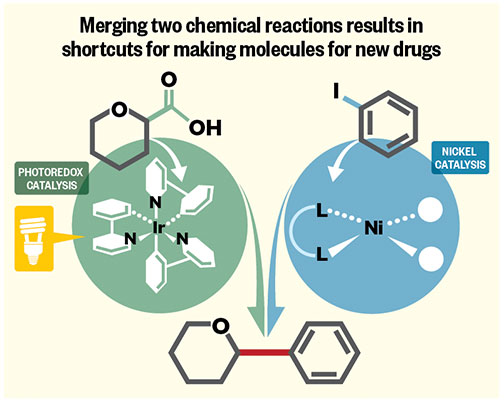
Associate professor Abigail Doyle and her team focused on using metal nickel to create links between carbon molecules, which are the fundamental building blocks for molecule creation. Professor David MacMillan’s lab worked on photoredox chemistry, which uses light to selectively switch on molecules that boost the speed of a chemical reaction. Putting the two concepts together, the chemists came up with a reaction that can join molecules that previously could not be directly linked. They also produced a reaction that created sought-after molecules in a single step, rather than requiring six or seven separate, consecutive reactions to be completed — a process that can take weeks, says Doyle, who was awarded the 2014 National Fresenius Award for most outstanding chemist under 35 in the United States.
One of the benefits this provides for the pharmaceutical industry is the ability to use carboxylic acids — a ubiquitous chemical in biological compounds — as a handle to connect two different molecules. “There were not previously known ways to make this happen,” says MacMillan.












No responses yet