
In March 2018, Susan Schaefer ’01 was nursing her 8-month-old son, Finn, and noticed something odd about one of his eyes. “One side was red, like asymmetric pink eye,” she says. Schaefer took Finn to the pediatrician and then an ophthalmologist, where an ultrasound revealed a large mass in his eye.
They were then directed to the Memorial Sloan Kettering Cancer Center in New York City for more testing. It turned out that Finn had an extremely rare and malignant cancer — uveal melanoma. “The oncologist said he had never seen anything like this in a child so young,” Schaefer says. Weeks later, that oncologist removed Finn’s eye. Schaefer and her husband hoped they were in the clear but had to take their toddler in for a visual exam every six months. “Even though his eye which contained the tumor had been removed, we didn’t know if any cancerous cells could be lurking in the eye socket,” she says.
However, on a visit in May 2021, Schaefer was offered an alternative to the exam. “The doctor told me there was another test that could tell if the cancer returned by looking for tumor DNA in his blood.” When the test came back negative, “it allowed me to let go of a lot of stress and worry in a way I wasn’t able to after the visual exams,” Schaefer says.
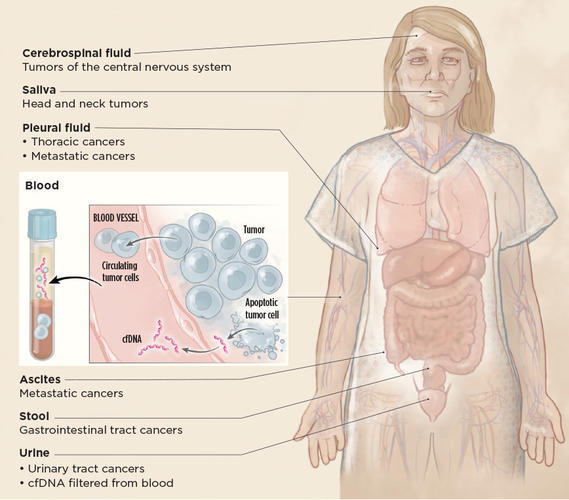
The blood test used on Schaefer’s son is known as a liquid biopsy, a relatively new approach that has seen rapid advances and adoption in cancer medicine over the past decade.
Scientists have long known that DNA fragments float freely in the blood, mainly from dying cells at the end of a normal cell cycle. In tumors, the cell cycle is on fast forward, with tumor cells proliferating and dying at dizzying rates, often resulting in detectable cell-free DNA from the tumor in the blood of cancer patients. About 10 years ago, when DNA sequencing became more sensitive and affordable, oncologists began to see liquid biopsy as an alternative to a standard biopsy: It promised a less invasive procedure to get data that could guide cancer treatment in real time. Then, in 2015, a landmark study showed that the suspicious DNA patterns in several pregnant women undergoing prenatal testing, via bloodwork, were due to cancer. The idea that perhaps one could screen for cancer, before symptoms and with a blood test, took hold.
In a 2018 article in The New England Journal of Medicine, Dr. Ryan Corcoran ’99 called an early cancer detection blood test the “holy grail.” While routine screening is available for a handful of cancers (breast, colon, lung, prostate, and cervical), 600,000 people die from cancer every year in the United States, and 80% of those deaths occur due to cancers with no screening tests. Now, the first tumor DNA blood test, GRAIL’s Galleri test, with the potential to detect a variety of cancers before patients have symptoms, could receive FDA approval in early 2026.
“It is remarkable how much progress the field has made over the past few years toward blood-based early detection of cancer, and this could lead to a profound reduction in cancer mortality,” says Corcoran, an oncologist and researcher at Massachusetts General Hospital and an associate professor of medicine at Harvard.
PAW spoke with Corcoran and several other Princetonians who are using liquid biopsy about how it is changing cancer detection and care, and what the future looks like for this groundbreaking technology.
After a stint interning for Merck in 1994, set up by a connection through his grandmother, who was somewhat of a local celebrity as the owner and hostess of the Lido Diner on Route 22 in Springfield, New Jersey, Corcoran majored in molecular biology at Princeton and went on to get his M.D.-Ph.D. at Stanford in 2006. “I loved medicine and research, and wanted to live on the edge between them,” says Corcoran.
Corcoran joined the faculty at Massachusetts General Hospital and Harvard in 2012. In 2014, fueled by a curiosity about how cancers resist treatment, his lab was one of the first to explore the potential of liquid biopsy. “Often, the physical tumor biopsies didn’t work,” he says. Because a tumor’s genetic variations can be so heterogeneous throughout the tumor, a physical biopsy on one part of the tumor can often miss the big picture. “With a standard biopsy,” he says, “it was like if Darwin had gone to the Galapagos and looked at the variation in finches on one island. A liquid biopsy lets us see all of the islands,” and theoretically, all of the DNA variation present in the tumor.
In addition, because tumors can be remarkably diverse in their genetic makeup, certain variants within the tumor lie in wait. Throughout a course of standard cancer treatment — chemotherapy and radiation, for example — the cells that are resistant to treatment can proliferate and eventually dominate, letting the cancer persist. “Unfortunately, every cancer becomes resistant,” Corcoran says.
Corcoran’s group at Massachusetts General Hospital and Harvard used liquid biopsy on gastrointestinal cancer patients to see how their tumors were becoming resistant during treatment. The first few patients were part of a clinical trial for a new drug; Corcoran’s group kept seeing DNA mutations arising in the exact gene the drug was designed to target. More and more patients showed mutations in this gene as they became resistant to treatment. “We were able to see exactly what was driving two-thirds of the resistance to this drug,” Corcoran says.
The work helped accelerate the development of new drugs that could tackle the resistance, in collaboration with several drug companies. “In some cases, we were able to make very precise, even predictive treatment decisions, matching patients to therapies that could potentially overcome treatment resistance” based on the specific DNA mutations detected, Corcoran says.
At the Inova Schar Cancer Institute in Fairfax, Virginia, medical oncologist Dr. Lauren Mauro ’02 learned of liquid biopsy several years ago, but, she says, “We started using it in breast cancer routinely in the last few years when it became more clinically available and accepted as an accurate way to check for mutations that we could target with our newer drug therapies.” She says liquid biopsy is also useful in situations where a tissue biopsy poses a risk to the patient. In the past, a needle biopsy was needed to investigate a tumor in places like the lungs, liver, or bones. “But,” Mauro says, “this is an invasive procedure requiring anesthesia, holding of blood thinners, a ride home from the hospital, and time off of work. It also carries a small risk of infection, bleeding, and pain.” In many cases a liquid biopsy can be done instead. “A simple blood draw is by far easier, quicker, and safer for the patient. This has been a game changer for us,” she adds.
In her lab at the Yale School of Medicine, oncologist and researcher Dr. Anne Chiang ’87 uses liquid biopsy routinely in lung cancer patients at diagnosis. She says that while tissue biopsies remain the gold standard, they take time, and “I do the blood test at the same time as the tissue biopsy, because I can see the mutations in the tumor faster, and sometimes because we find out we didn’t get enough cells in the tissue biopsy.” This way, the time to treatment is quicker and the liquid biopsy can guide treatment and validate the results of the tissue biopsy. Chiang continues to use liquid biopsy throughout treatment. “Even if a patient is doing really well on a treatment targeted against one mutation, they often develop resistance. So, we always do a liquid biopsy at that time if it looks like the treatment isn’t working.”
Yet, as many patients, families, and their medical teams know all too well, even when cancer appears beaten, it can return. In lung cancer patients, ongoing trials are exploring how to use liquid biopsy to determine if a patient is really in remission or if cancer cells are again proliferating, says Chiang. A liquid biopsy may be able to detect such “residual disease” — microscopic cancer — before a tumor shows up on an image. “By the time you see a tumor on a CT scan, it’s about one centimeter in diameter, which is about a billion cells,” says Corcoran. “At that point, the odds of one or more cancer cells being resistant to whatever therapies we have to offer is high. You really need to intervene when there are fewer cancer cells present, before you see the tumor on a scan.”
In 2020, in collaboration with a group at Memorial Sloan Kettering, Corcoran’s group launched the first clinical trial in the U.S. — the ACT3 trial, funded by Stand Up to Cancer — to adapt postsurgical therapy based on the detection of residual disease in colon cancer patients.
After medical school, Dr. Chloe Atreya ’98 chose UCSF for her oncology fellowship so she could work in the lab of her Princeton senior thesis adviser, Kevan Shokat. She is now a gastrointestinal oncologist at the UCSF Helen Diller Family Comprehensive Cancer Center. She says that in her practice, liquid biopsy has changed how she thinks about colon cancer stages and treatment. For example, Stage 2 colon cancer traditionally has been defined by a tumor that has not yet spread to the lymph nodes and typically is cured by surgery. However, if the tumor has certain high-risk features, doctors may recommend chemotherapy after surgery.
This thinking changed in 2019 when a clinical trial in Australia showed that if, after surgery, the liquid biopsy came back positive, there was nearly a 100% chance of cancer recurrence, even in cases where the tumor lacked those high-risk features. “I now routinely use liquid biopsy with my patients who have Stage 2 colon cancer,” Atreya says.
“It used to be that we chose the cancer regimen based on the tumor location,” says Dr. David Henry ’70, “but the staging in colon cancer is imperfect. With liquid biopsy, we now know there might be Stage 2 patients who need chemotherapy and Stage 3 patients who don’t. Liquid biopsy is redefining what the stages mean in colon cancer.”
An oncologist since 1981 and now practicing at Pennsylvania Hospital in hematology and oncology, Henry says he remembers when cancer treatment was one size fits all, and then in the early 2000s, it changed with the ability to see genetic markers of tumors. “It’s an amazing time,” he says.
Dr. Julia Beaver ’01 says that better detection of residual disease has great public health implications. “Right now, only certain patients may benefit from additional treatment — for example, chemo or radiation — after surgery [to remove a tumor]. More patients than necessary often receive additional treatment to account for the chance the cancer could come back,” says Beaver, who briefly played professional squash after graduating from Princeton before training to become an oncologist. She then moved to the FDA in the oncology division, most recently as chief of medical oncology at the Oncology Center of Excellence, until her current role as senior vice president of clinical development at Treeline Biosciences. “Right now, there are multiple ongoing trials testing whether tumor DNA can be used to select patients with remaining residual disease after surgery and if they would benefit from additional treatment,” she says.
Last April, an FDA advisory committee voted unanimously to allow residual disease to be used as an endpoint in drug trials for multiple myeloma. Such a move may expedite drug development for patients with multiple myeloma and can be used as a framework to explore the utility of residual disease in other cancer subtypes, says Beaver.
So, what about the holy grail: a blood test to tell if a person has cancer before they have any symptoms? Such tests are already available for specific cancers. Last July, a blood test for colon cancer, Guardant’s Shield test, was approved, and more recently, the FDA approved an updated form of Cologuard, which looks for DNA signatures of colon cancer in a person’s stool. While a colonoscopy remains the recommendation for colon cancer screening, only about half of the population avails itself of the test, according to studies.
“If you could reach 90% to 95% compliance with a blood test,” says Corcoran, “then you could potentially catch more. You can tolerate a higher false positive rate if, after a positive result, the next step is a colonoscopy.”
“You don’t know in a traditional needle biopsy if you’ve sampled all of the sites. It would be nice for a patient to know their actual risk … . A liquid biopsy might be able to give a patient a more accurate prognosis.”
Graham Read ’15
Graham Read ’15 is working on developing a new liquid biopsy for prostate cancer, based on RNA, in his position as a postdoctoral fellow at the University of Illinois Cancer Center.
For decades, prostate cancer has been diagnosed through a different kind of liquid biopsy, one that looks for the protein prostate specific antigen (PSA) in the blood, a test that has been riddled with false positives, since a benign prostate can also pump out lots of PSA. Another problem with prostate cancer diagnosis is distinguishing the more aggressive kinds from the slow-growing ones that might not cause harm for decades. “If we can’t tell the difference between a more aggressive or less harmful cancer,” Read says, “then the idea is, you treat as if it’s the worst-case scenario. But that results in a lot of people with less aggressive tumors getting aggressive treatment.”
Read is trying to identify RNA signatures of the more aggressive cancer. He is looking at very small RNA molecules — called microRNAs — as potential markers to identify the more harmful cancer. “At one time, people thought microRNAs were junk,” says Read. “Their usefulness in molecular biology was only recently recognized” — in fact, the discovery of microRNAs won the 2024 Nobel Prize in Physiology or Medicine — “and now they could turn out to actually guide cancer treatment.”
Prostate cancer is different from other cancers in that there are almost always multiple tumor sites on the prostate, Read explains. “You don’t know in a traditional needle biopsy if you’ve sampled all of the sites,” he says. A liquid biopsy, by contrast, gives a comprehensive view. “It would be nice for a patient to know their actual risk. No one wants to hear, ‘You have cancer,’ and the default idea is, I should do everything I can, including aggressive treatment that can be hard on the body. A liquid biopsy might be able to give a patient a more accurate prognosis.”
Nick Papadopoulos, a professor of oncology and pathology at the Johns Hopkins University School of Medicine, and the co-founder of two companies focused on early cancer detection, praises the promise of an early detection blood test for multiple cancers. “When we catch cancers at Stage 1,” as such a test could do, “the chances of survival are good. We can think about curing people, not just about prolonging life,” he says. But, Papadopoulos continues, the bar for such a test is high. If a person received a positive result, the next step would be some kind of imaging to pinpoint the location of the tumor. “You can’t have a lot of false positives,” says Papadopoulos, that would send people off for expensive and anxiety-provoking imaging and other procedures, some of them invasive, which they ultimately don’t need.
Daniel Notterman, a professor in molecular biology at Princeton, says liquid biopsy tests could be especially useful for pancreatic and ovarian cancer, both typically not detected using conventional methods until they have spread throughout the body and a cure is out of the question. The Wall Street Journal recently described such a case, in which someone detected pancreatic cancer early enough to treat via the Galleri test.
GRAIL’s Galleri test promises to detect over 50 cancers, many of which are the aggressive, lethal cancers that lack other screening methods, and is scheduled for FDA review in early 2026. Galleri is available now with a prescription and has been offered to eligible Princeton University faculty and staff over age 40 as part of a pilot program with GRAIL for the past two years. Looking ahead, Notterman says, these tests will probably become common “when they have been tuned to detect tumors early enough so that they are very easily removed and treated, in the same way that colon cancer screening allows the removal of polyps before they progress to advanced cancer.”
Eric Klein, a former professor of urology at the Cleveland Clinic and a scientist at GRAIL, is undeterred by a vocal complaint by physicians, which is what if the test is positive but no tumor shows up on a follow-up imaging test? “While it may be that no cancer is present,” he says, “it could also be a case in which a more sophisticated follow-up might find it,” he says, citing an example of a patient in a trial in whom an unusual kind of cancer was found in the person’s small bowel. “We are learning how to look and discover at the same time, and find things we usually don’t see,” he says. Furthermore, he adds, the cancers that shed detectable levels of DNA into the blood are by nature more aggressive. “It’s unlikely you’ll see a cancer detected by Galleri that is nothing to worry about,” he says.
For certain, blood tests that detect cancer DNA are rapidly changing cancer care and stand to transform cancer detection in the near future. Papadopoulos recalls the first screening tests for the BRCA gene, which dramatically increases one’s chance of developing breast cancer. “All we heard at first was ‘No! Those tests will never take hold!’ and look, it’s everywhere now.” Beaver agrees. “These tests have even more promise than what we are seeing in current applications,” she says. “These tests are here to stay.”
Susan Reslewic Keatley ’99 majored in chemistry and is now a writer and host of the podcast Science Fare.






3 Responses
Kenneth Offit ’77, M.D., Charles Sawyers ’81, M.D.
9 Months AgoDr. Michael Berger ’01’s Contributions to Liquid Biopsy
The recent feature “A New Way To See Cancer” profiled the outstanding scientific and clinical work of Dr. Ryan Corcoran ’99, as well as other alumni including Dr. Julia Beaver ’01, Graham Read ’15, Dr. David Henry ’70, Dr. Chloe Atreya ’98, Dr. Anne Chiang ’87, Dr. Lauren Mauro ’02 and others who have been leaders in the use of DNA sequencing and “liquid biopsy” as a new advance in cancer diagnosis. To that list we would like to add Dr. Michael Berger ’01 whose team developed the liquid biopsy test MSK-ACCESS using ultra-deep sequencing of 146 key cancer-associated genes based on an FDA cleared panel he developed at Memorial Sloan Kettering (MSK) called MSK-IMPACT. These panels detect changes in the tumor genome that can be targeted for therapy. MSK IMPACT has now been used to care for over 125,000 individuals with cancer, and has had a broad scientific impact on our field. At the most recent meeting of the American Association of Cancer Research, Mike was honored with the “Team Science Award” for leading this pathbreaking work.
James Corsones ’75
11 Months AgoHuge Advance for Cancer Treatment
This is fascinating. After 38 years in internal medicine, I've seen the practice of oncology dramatically change. When I was a new clinician, the treatment might have cured the cancer, but frequently killed the patients with toxicity. Anything to direct therapy more specifically will be a huge boost to the cancer community. As rightly pointed out, the biggest issue will be a positive liquid biopsy and no detectable disease but that should be able to be worked out with proper research. This is huge from a public health and expenditure standpoint.
Randy Mamiaro ’80
11 Months AgoA Follow-Up on Medical Research Funding
I enjoyed reading, in the March 2025 issue of PAW, Susan Reslewic Keatley ’99’s piece on cancer research being conducted by Princeton alumni. It was an insightful and informative article. I was a bit surprised not to see Dr. Monica Bertagnolli ’81 included as one of the alumni on the forefront of cancer research. Dr. Bertagnolli served as the director of the National Cancer Institute as well as the director of the National Institutes of Health and has had a distinguished career in clinical oncology.
Perhaps an article focusing on policy issues associated with government support and funding (or lack thereof) for important and life-saving cancer and other medical research is in order. The views of Dr. Bertagnolli and other Princetonians with expertise in medicine and public policy would be a welcome follow-up to Ms. Keatley’s feature.