Researchers Find a Sustainable and Affordable Way to Harvest Clean Water
‘If we really put effort in making this environment more green, more sustainable, we can do it,’ says Xiaohui Xu
Xiaohui Xu has a bold ambition: to improve global water quality and reduce plastic pollution. Her five years of research in Princeton’s chemical and biological engineering department — spanning from flexible batteries to sustainable plastics derived from shrimp shells — could restore a greener ecosystem for the planet.
Xu, who was studying under Princeton’s Presidential Postdoctoral Fellowship in Professor Rodney Priestley’s lab, focuses on making hydrogels to address challenges in water purification and energy storage. These flexible gels — a Jello-like network of polymers bonded to water — are used in everything from drug delivery to adhesives. Xu, now a visiting research scholar, has her eye on environmental applications, such as filtering water contaminants, and developing more eco-friendly hydrogel structures.

With more than 2.2 billion people who lack access to safe drinking water, water stress is a growing threat to health, education outcomes, and food security across the world. The United Nations, which observed World Water Day on March 22 to highlight the importance of fresh water, predicts that 700 million people will be displaced by severe water scarcity by 2030.
“We do not have that experience in our daily life,” Xu says. But the water crisis reaches high-GDP countries, too, where per- and polyfluoroalkyl substances, also known as PFAS or “forever chemicals,” contaminate water and soil. PFAS toxins released into the environment from industrial waste and consumer products such as food packaging have been linked to immune system and liver damage, cancers, thyroid disease, and other health issues including birth and developmental defects. Some forever chemicals linger in rivers and on mountaintops for upward of 1,000 years or more.
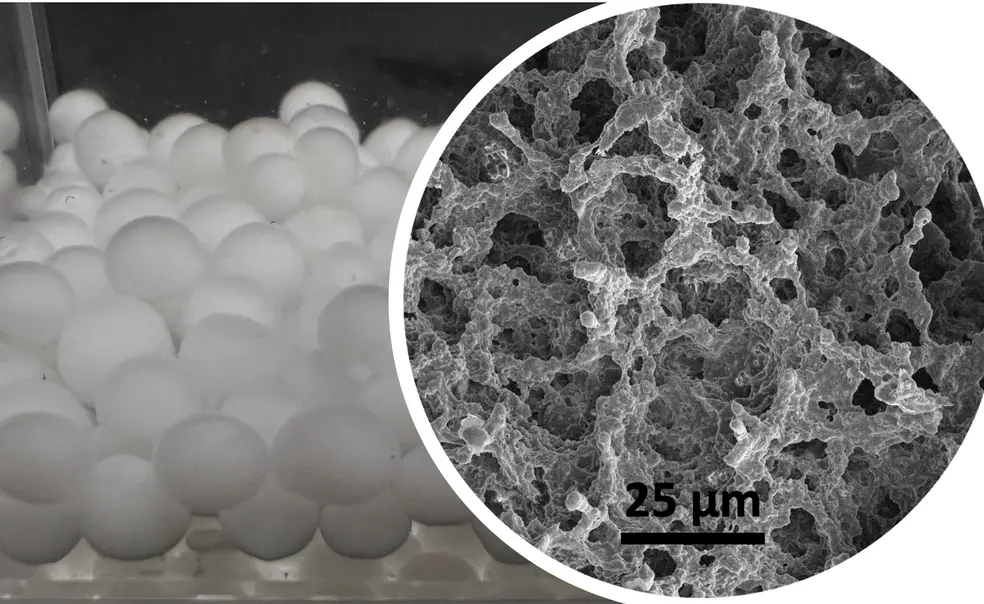
After completing her master’s on super-absorbent hydrogels, Xu’s experiences seeing pollution in the Passaic River in New Jersey — compared to say, a clean lake in Colorado — prompted her to explore gel solutions. Xu’s first project at Princeton involved creating a black hydrogel that absorbs water for purification and is heated by sunlight. When it reaches 32 degrees Celsius (89.6 degrees Fahrenheit), the gel undergoes a phase change and becomes hydrophobic, expelling the water. But a challenge remained, Xu says. The large size of the hydrogels slowed water absorption time. Her current work aims to make a smaller, more uniform gel that speeds the process: a novel porous, gel bead structure.
The open-pore morphology not only absorbs water quickly but also releases it within several seconds. To construct the beads and achieve this rapid response, Xu relies on the chemistry behind oil and water separation. After dissolving the gel’s monomer units in water, she suspends the material in an oil bath. When the polar monomers (N-Isopropylacrylamide) link to form long polymer chains, they remain in droplet shapes because they do not mix with oil. This technique creates a “very uniform, very beautiful” array of gel beads, Xu says.
Labs around the globe are researching PFAS clean up, but Xu’s gel beads deliver an easier, less energy-intensive process to absorb toxins. Other researchers have used metal-organic frameworks (MOFs), which often degrade slowly but are expensive and challenging to produce beyond laboratory scale. Another approach involves using ionic flurogel resins to absorb PFAS, but the chemicals are not environmentally friendly.
Besides improving efficiency through its high surface area, the new beads incorporate biodegradable cellulose instead of chemically synthesized polymers. The use of hydroxypropyl cellulose provides more hydrophobic sites: Water-fearing groups in the gel attract other water-adverse groups in PFAS, catching contaminants.
Cellulose boasts several advantages: cheaper production cost, improved mechanical qualities, and an abundant, sustainable alternative to traditional absorbents. Compared to nanoparticles like active carbon, the gel beads are also large enough to remove from the water to avoid secondary contamination — and can be reused.
In the future, hydrogels could benefit the design of flexible batteries. A zinc-ion battery with a gel electrolyte would be an environmentally friendly replacement for batteries based on lithium, which is costly to extract from sea water and strains limited energy resources, Xu says.
A multitasker, she plans to continue applying her dual studies in environmental and chemical engineering to better the Earth in multiple, intersecting ways. “I’m lucky to have both backgrounds,” says Xu, who began teaching at Rowan University this year.
That includes advancing her team’s other project on sturdier, recyclable hydrogels, published September 2023 in Journal of the American Chemical Society Au. Improvements last autumn in temperature tolerance and conductivity make them even more viable as an eventual swap for nondegradable plastics, Xu says — which would help protect oceans and rivers. But she’s also committed to enhancing water quality for local communities. Xu’s long-term goal is to not only absorb water contaminants but also to degrade them.
“If we really put effort in making this environment more green, more sustainable, we can do it.” Xu says. “From the technology side, people are so smart they can make a lot of impossible things become possible.”











No responses yet